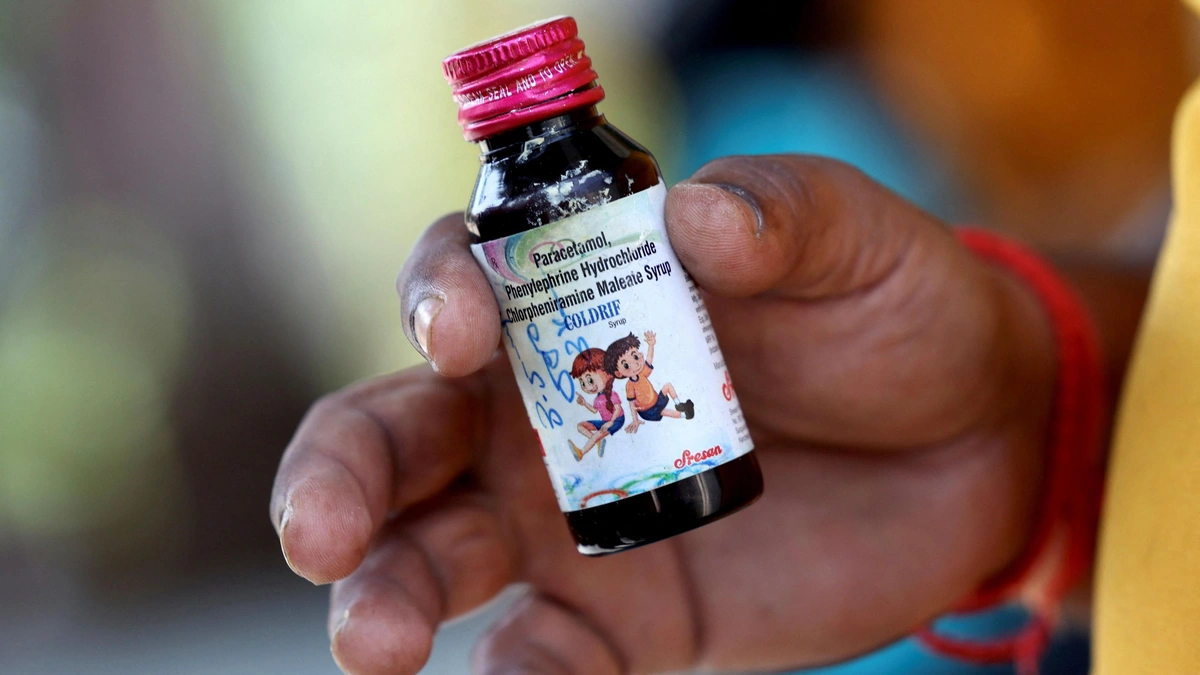
WHO Warns Against 3 Indian cough syrups Due to Toxic Substances
Okay, let’s be real. When the World Health Organization (WHO) throws down a warning about something, especially something as common as cough syrups , we need to sit up and listen. This isn’t just about a recall; it’s about our health, our families, and understanding the why behind these warnings. It is important to note that substandard medicines can cause health issues. So, what’s the deal, and why should you in India specifically care?
The Alarming Details: What’s Really in These cough syrups?

The headline is scary enough: “Toxic Substances.” But what does that even mean? We’re talking about contaminants like diethylene glycol and ethylene glycol. These aren’t ingredients; they’re industrial solvents that can cause serious kidney damage, neurological problems, and in the worst cases death. Think of it like this: you’re expecting a soothing remedy for a cough, and instead, you’re getting a dose of something that could land you in the hospital. This is not acceptable and it is necessary to understand the potential adverse effects .
These toxic substances can cause serious health risks, especially for children. Symptoms of poisoning include:
- Nausea
- Vomiting
- Abdominal pain
- Kidney problems
- Neurological issues
The WHO’s warning specifically targets three Indian-made cough syrups . But , here’s the thing: these products may have been distributed beyond India, ending up in other countries through informal markets.
Why This Matters So Much to Us in India
Let’s be honest, India has a massive pharmaceutical industry. We’re the “pharmacy of the world,” producing affordable medicines for millions. But with that scale comes responsibility. Incidents like these erode trust both within India and internationally. They raise serious questions about quality control, regulation, and the effectiveness of our drug monitoring systems. And that is why it’s necessary to understand the pharmaceutical quality .
I initially thought this was an isolated incident, but then I realized the potential ripple effect. If people start questioning the safety of Indian-made medicines, it could hurt our economy, damage our reputation, and, most importantly, put lives at risk.
Here is an external link aboutcough syrups.
How Can We Protect Ourselves and Our Families?
Okay, so we know the problem. What can we actually do about it? This is where it gets practical. A common mistake I see people make is blindly trusting labels. Here’s what you need to do:
- Be extra cautious with medications for children: Kids are more vulnerable to the toxic effects of these substances. Always consult a doctor before giving any medication to a child.
- Check for official alerts and recalls: The Drug Controller General of India (DCGI) is the authority that issues alerts about unsafe medicines. Keep an eye on their website and news reports.
- Buy from trusted sources: Stick to reputable pharmacies and avoid buying medicines from unverified online sources or street vendors.
- Report any adverse effects: If you suspect that a medicine has caused a problem, report it to your doctor and the relevant authorities. The adverse drug reaction monitoring system can help.
Let me rephrase that for clarity: knowledge is power. The more we know, the better equipped we are to make informed decisions. The government regulations play a crucial role in ensuring the safety of pharmaceuticals.
Beyond the Immediate Crisis | A Call for Systemic Change
This incident isn’t just about specific cough syrups ; it’s a symptom of deeper issues. We need stronger regulations, stricter enforcement, and more transparency in the pharmaceutical industry. The central government needs to invest more in quality control and monitoring. The drug manufacturing companies have a crucial role to play.
What fascinates me is the potential for technology to help. Imagine a system where you can scan a QR code on a medicine package and instantly verify its authenticity and safety. Blockchain technology could play a role in tracking medicines from manufacturing to distribution, making it harder for substandard products to enter the supply chain. It also highlights the importance of medicine safety .
This is not just a problem for the government or the pharmaceutical companies to solve. It requires a collective effort from doctors, pharmacists, consumers, and the media. And , here is an internal link about pharmaceutical companies .
FAQ | Your Burning Questions Answered
What specific cough syrups are included in the WHO warning?
The WHO has identified three specific Indian-made cough syrups as being of concern. It is important to refer to the official WHO alert for the specific product names and manufacturers.
Where can I find the official WHO alert?
You can find the official WHO alert on the WHO website in the medical product alerts section. Search for the relevant alert based on the date and product type.
What should I do if I have given my child one of these cough syrups?
Seek immediate medical attention. Explain the situation to your doctor and bring the medicine packaging with you.
How can I report a suspected adverse drug reaction in India?
You can report adverse drug reactions to the National Pharmacovigilance Programme of India (NPvPI). Information on how to report can be found on the website of the Indian Pharmacopoeia Commission (IPC).
Are all Indian-made cough syrups unsafe?
No, this warning applies to specific products identified by the WHO. However, it’s a reminder to be vigilant and buy medicines from trusted sources.
What is the Indian government doing to address this issue?
The DCGI is investigating the matter and working with the manufacturers to take corrective action. They may also issue recalls for the affected products.
Ultimately, this WHO warning is a wake-up call. It’s a reminder that we can’t take our health for granted. We need to be informed consumers, demand quality and transparency, and hold the pharmaceutical industry accountable. It’s time to have a serious conversation about medicine regulations and how we can ensure that the medicines we take are safe and effective. The key here is to promote public health by verifying the authenticity of medicines and report any suspicious activity.