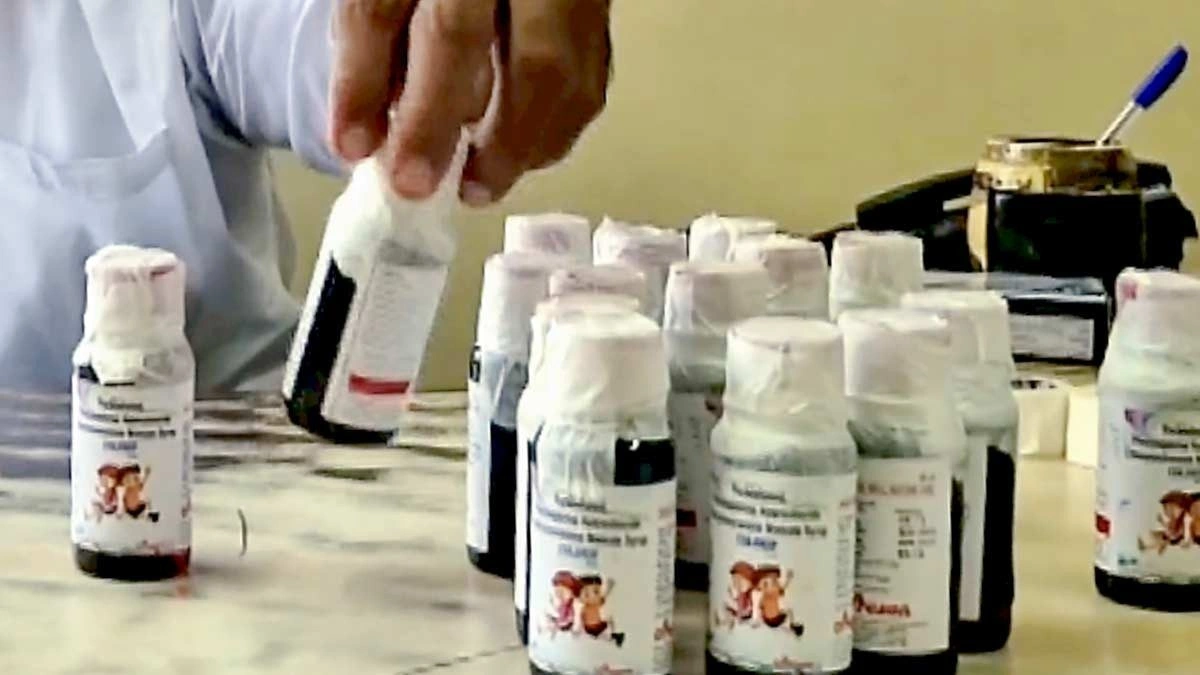
Kerala Prohibits Sale of Drugs from Disputed Cough Syrup Manufacturer
The news broke like a monsoon cloudburst: Kerala’s drug control department has put the brakes on the sale of medications manufactured by a cough syrup maker currently under scrutiny. But here’s the thing – what does this really mean for you, the person standing in a pharmacy, or the parent reaching for that familiar bottle when your child is coughing? Let’s dive in, because it’s more nuanced than a simple headline suggests.
Why This Kerala Drug Ban Matters More Than You Think
It’s easy to skim past a headline about a Kerala drug ban and think, “Okay, good for them.” But this isn’t just about one state and one manufacturer. This ripples outward. It’s a crucial reminder of the layers of oversight (or lack thereof) in the Indian pharmaceutical industry. What fascinates me is the potential for a domino effect. If one state finds issues serious enough to warrant a ban, what about others? Are they doing their due diligence?
The implications extend beyond just cough syrups. This incident shines a spotlight on the quality control mechanisms in place, or rather, the potential cracks within them. Think about it – we trust medications to make us better, not worse. And when that trust is shaken, it erodes confidence in the entire system. That’s why understanding the why behind this ban is so important. Quality and safety checks are essential to prevent similar incidents in the future.
But, it’s also not a reason to panic. It’s a reason to be informed, to ask questions, and to demand transparency. According to the official notifications, the Kerala drug control department is taking proactive steps to ensure public safety. The specific reasons cited often involve deviations from established manufacturing practices or the presence of contaminants. However, while this may be an isolated incident, it brings to light the importance of vigilance.
Deciphering the Details | What Drugs Are Affected?
The immediate question, of course, is which specific drugs are affected by this ban? Details matter. While the official notification lists the manufacturer, pinpointing the exact medications on shelves can be trickier. Here’s where your own diligence comes in. Check with your pharmacist. Don’t hesitate to ask if the medication you’re purchasing is from the manufacturer in question. Cross-reference the batch number with any public advisories issued by the drug control authorities . I initially thought this was straightforward, but then I realized the average person doesn’t have time to deep dive into the specifics.
And, honestly, information can be scattered. Official announcements may be in technical language. News reports might not always be comprehensive. So, where can you reliably find information? Look for official press releases from the Kerala government and reputable news sources that cite those releases. The more specific the information, the better. Be wary of social media rumors and stick to verified facts. The cough syrup contamination incidents are serious public health concerns.
Navigating the Pharmacy | A Practical Guide for Consumers
Okay, so you’re at the pharmacy. You need a cough syrup. How do you navigate this situation practically? Here’s the thing: don’t be afraid to ask questions. A good pharmacist is your ally in this. Ask them if they are aware of the ban and if any of the medications they are dispensing are affected.
A common mistake I see people make is blindly trusting the brand name. Dig deeper. Look at the manufacturer’s name and address on the packaging. If it matches the one under scrutiny, consider alternatives. Talk to your doctor about other options. Natural remedies, like honey and lemon for a cough, can sometimes provide relief. What fascinates me is how often we forget the simple, time-tested solutions in our rush for quick fixes.
The Broader Picture | Regulation and Responsibility in the Pharma Sector
This cough syrup ban raises a much larger question: how robust is the regulatory framework governing pharmaceutical manufacturing in India? And what about accountability? It’s not just about catching errors; it’s about preventing them in the first place. What measures are in place to ensure that manufacturers adhere to Good Manufacturing Practices (GMP)? What are the consequences when they don’t?
Let’s be honest, the system isn’t perfect. There are loopholes, challenges with enforcement, and instances of negligence. But, incidents like this one can serve as a catalyst for change. They can prompt stricter regulations, increased oversight, and a greater emphasis on quality control. It’s about holding manufacturers accountable, protecting public health, and restoring trust in the medications we rely on. The pharmaceutical industry needs to prioritize patient safety above all else.
Taking Action | What You Can Do to Stay Informed and Safe
Ultimately, staying informed and safe is a shared responsibility. As consumers, we need to be proactive. Check for updates from regulatory bodies. Don’t self-medicate without consulting a doctor. Report any adverse reactions to medications. Ask questions, demand transparency, and hold manufacturers accountable.
As per guidelines, public awareness campaigns play a vital role in educating people about drug safety. These campaigns can provide information on how to identify substandard or counterfeit medications, how to report adverse drug reactions, and how to make informed choices about their healthcare. Public awareness is key to ensuring that people can protect themselves from the risks associated with substandard or counterfeit drugs. Public health is a shared responsibility that requires the active participation of individuals, healthcare professionals, and regulatory authorities.
FAQ
What specific cough syrups are affected by the Kerala ban?
Refer to official notifications from the Kerala government and reputable news sources for the most up-to-date list of affected medications. Check the manufacturer’s name and batch number on the packaging.
What should I do if I have a cough syrup from the affected manufacturer?
Consult your doctor or pharmacist immediately. Do not continue using the medication. They can advise you on safe alternatives.
How can I report a suspected issue with a medication?
Report any adverse reactions or concerns about medication quality to the drug control authorities in your state or to the national pharmacovigilance program.
Where can I find reliable information about drug safety?
Official websites of drug regulatory bodies (both state and national), reputable news sources, and your doctor or pharmacist are good sources of information.
Is this a widespread problem, or is it limited to this specific case?
While this specific ban is related to one manufacturer, it highlights broader concerns about quality control in the pharmaceutical industry. Vigilance and awareness are always important.
What if I already consumed the drug?
Monitor yourself for any adverse reactions, and immediately consult your doctor. Provide them with the details of the medication you consumed.
This isn’t just about a drug ban in Kerala ; it’s about a larger conversation around trust, transparency, and the relentless pursuit of quality in healthcare. We need to keep asking the hard questions, holding the powerful accountable, and demanding a system that prioritizes our health above all else.